how do leucine zippers work
DNA Binding and Phosphorylation Regulate the Core Structure of the NF-κB p50 Transcription Factor. Leucines occurring in heptad repeats end up on the same sides of the helixes and are adjacent to each other in the stem of the Y the zipper region.
In the basic-region leucine-zipper domain flexible DNA-binding arms are juxtaposed by a two-stranded parallel coiled-coil motif called the leucine zipper.
. Leucine Zipper - Web Books Publishing. ZIP forms an amphiphilic α helical structure in which two residues that are separated by seven residues in sequence are located at nearly the same molecular surface in an α helix. A heptad repeat of leucine residues leucine zipper ZIP is an important sequence motif facilitating proteinprotein interactions.
Dimers leucine zippers and DNA-binding domains Transcription factors can be divided into classes on the basis of their mode of interaction with the target promoter sequence. How do leucine zippers work Tuesday March 15 2022 Edit Her she two been other when there all during into school time may years more most only over city some world would where later up such used many can state about national out known university united then made. A zipper track is made up of dozens of teeth each of which combines a hook and a hollow.
A structure referred to as the leucine zipper or simply as ZIP has been proposed to explain how a class of eukaryotic gene regulatory proteins works Landschulzetal1988Asegmentofthemammalian CCAATenhancer binding protein CEBP of 30 amino acids shares notable sequence similarity with a segment of the cellular Myc. On dimerization the leucine-zipper a helices form a parallel-coiled coil based on hydrophobic interfacial side-chain packing 55. The zipper is made up of 2 main parts.
The idea is to latch every hook on each of the two tracks into a hollow on the opposite track. These responses are usually originated by regulating the expression of relevant genes. We exploit this fact for identification of leucine zippers from sequence alone.
My Masters Work -. The leucine zipper is formed by amphipathic interaction between two ZIP domains. The leucine repeat in the sequence has been traditionally used for identification however with poor reliability.
Nature - Action of leucine zippers. The leucine zipper is a dimerization domain occurring mostly in regulatory and thus in many oncogenic proteins. The ZIP domain is found in the alpha-helix of each monomer and contains leucines or leucine-like amino acids.
DNA Binding and Phosphorylation Regulate the Core Structure of the NF-κB p50 Transcription Factor. Genetic physical and structural studies of the leucine zipper identify interactions that help determine the stability and specificity of dimerization and DNA binding. The coiled coil structure of a leucine zipper is required for dimerization and can be predicted with reasonable accuracy by existing algorithms.
The leucine zipper is an amphipathic a helix containing heptad repeats of Leu residues on one face of the helix and serves as a dimerization module. A leucine zipper aka leucine scissorsis a original three-d structural motif in proteins. Synonyms Leu Derived words.
BZIP basic leucine zipper transcription factors as one of the largest transcription factor regulatory families play very important roles in responses to these abiotic stresses. Introduction TheleucinezipperisthedimerizationdomainoftheB-ZIP basic-region leucine zipper class of eukaryotic transcrip- tion factors Vinson et al 1989. ZIP forms an amphiphilic α helical structure in which two residues that are separated by seven residues in sequence are located at nearly the same molecular surface in an α helix.
C6H13NO2 dispensibleof the inactive state 25 26 Octo Vol 278. They had been first described by means of Landschulz and collabora. Different protein domains responsible for DNA recognition have been identified.
In this review we discuss the leucine zipper structure which has been found in several nucle. The slide brings the two rows of teeth together to ZIP closed and then pulls them away again when you UNZIP. DNA-binding motifs formed from two alpha-helixes which intertwine for about eight turns into a coiled coil and then bifurcate to form Y shaped structures.
Leucine repeat but do not adopt the leucine zipper structure we shall refer to these as non. BZIP TFs could be activated by drought high salt and chilling damages. The leucine zipper is a proteinprotein interaction domain consisting of amphipathic a helices that dimerize in parallel either as homodimers or heterodimers to form a coiled-coil.
Leucine zipper is created by the dimerization of two specific alpha helix monomers bound to DNA. These amino acids are spaced out in each regions polypeptide sequence in su. The region of IKK- containing the leucine zipper-like motif was required both for homodimerization of IKK- and for formation leucine zippers.
A heptad repeat of leucine residues leucine zipper ZIP is an important sequence motif facilitating proteinprotein interactions. The DNA-binding residues are located in the bifurcated region of the Y.

Leucine Zippers Leucine Zipper Of The Yeast Activator Protein Gcn4 Download Scientific Diagram

Ijms Free Full Text Multiple Links Between Hd Zip Proteins And Hormone Networks Html

Pin On Athletic Aesthetic Outfits Men And Women
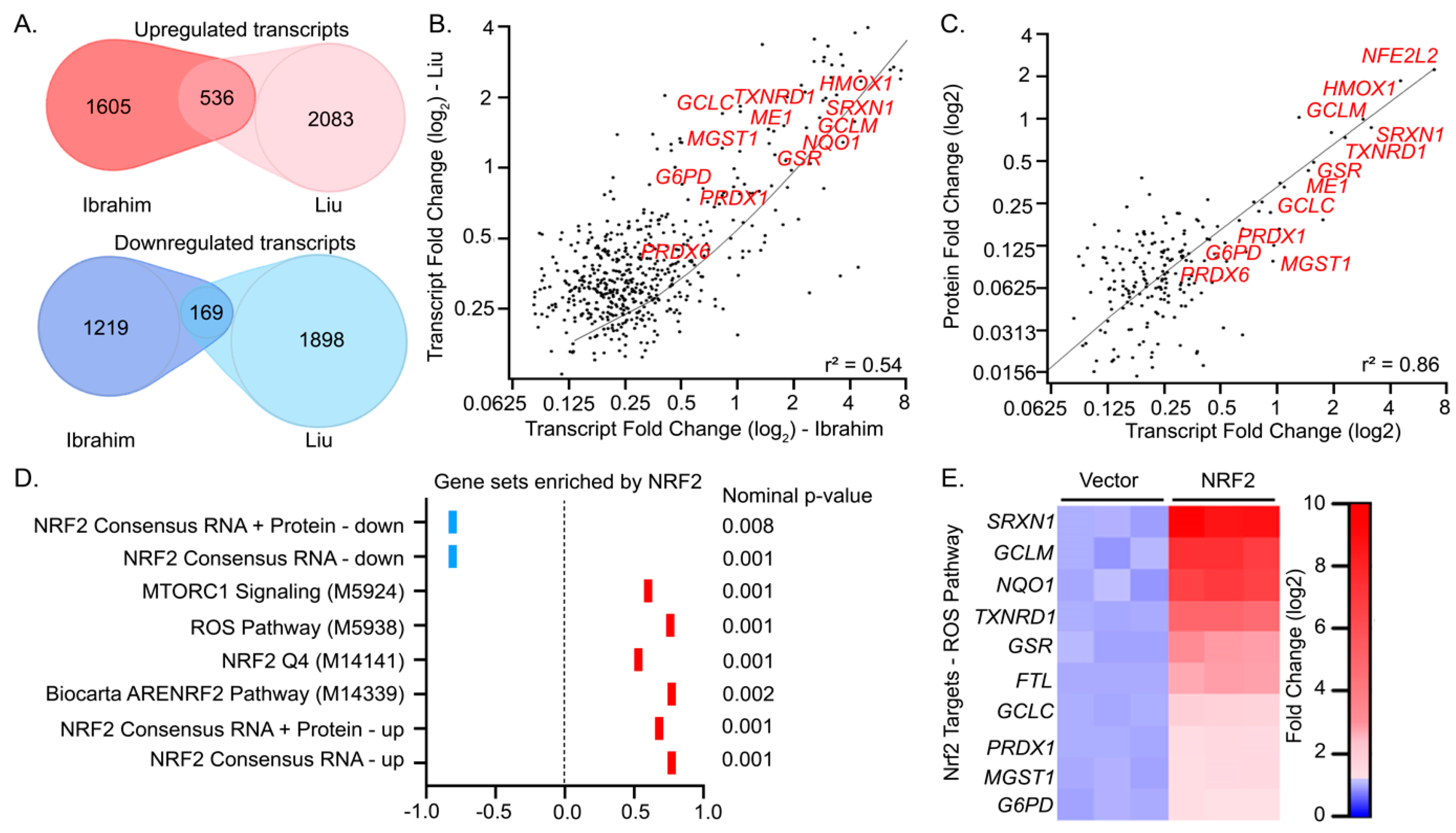
Antioxidants Free Full Text Defining The Functional Targets Of Cap N Collar Transcription Factors Nrf1 Nrf2 And Nrf3 Html

Basic Leucine Zipper Transcription Factor An Overview Sciencedirect Topics

Modelling Of The Interaction Of The Atbzip63 Helical Download Scientific Diagram
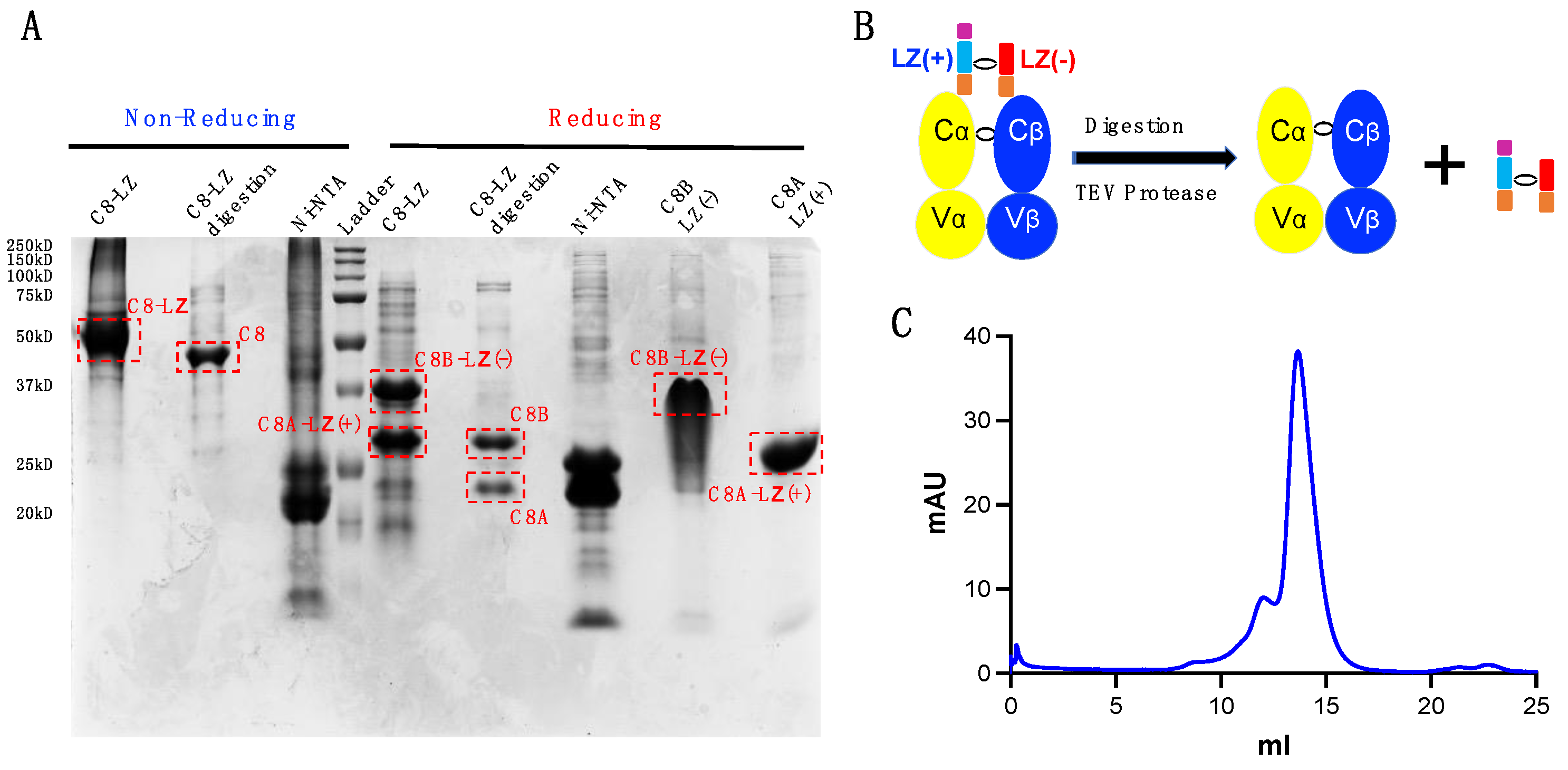
Cells Free Full Text A Leucine Zipper Dimerization Strategy To Generate Soluble T Cell Receptors Using The Escherichia Coli Expression System Html
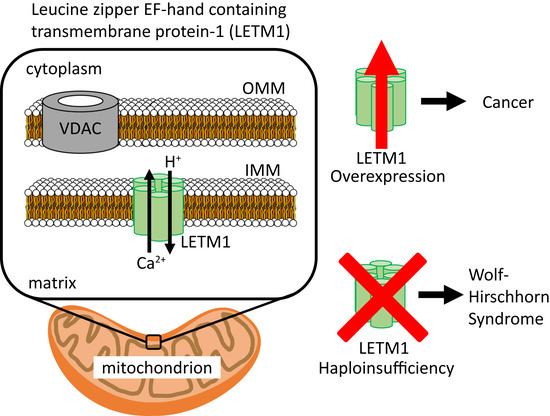
Ijms Free Full Text Molecular Mechanisms Of Leucine Zipper Ef Hand Containing Transmembrane Protein 1 Function In Health And Disease Html

Amino Acid Sequence Of 7 Human Leucine Zipper Regions Proteins Are Download Scientific Diagram





